Cross Reactivity, Gluten
19 Gluten Cross-Reactive Foods Myth Busted
19 Gluten Cross-Reactive Foods Myth Busted
Abstract
Numerous online blog articles report ”19 Gluten Cross-Reactive Foods.” Close inspection reveals questionable conclusions and significant overstatement and extrapolation from the original sources of information. Alternate conclusions presented within this blog article would call into question the existence of many blog-reported gluten cross-reactive foods. Lessons about rigorous research and reporting of scientific data can be learned.
KEYWORDS
There have been a number of warnings issued about ’19 Gluten Cross-Reacting Foods’ repeatedly featured on a variety of popular and trusted health and diet blogs. [ 1, 2, 3 ] The sheer number of articles that are circulating with the same information gives readers, naturopathic healthcare practitioners, patients, and the general public a sense of informational credibility and reliability.
The basic gist of these articles is that there are approximately 19 foods that “cross-react” with gluten— that these other foods can mimic gluten. This leads to your body reacting to these “cross-reactive foods” as if they were gluten, making these foods problematic for anyone who has a sensitivity to gluten (either Celiac disease or non-Celiac gluten sensitivity). In other words, if you are making antibodies to gluten, those same antibodies recognize other food proteins.
However, because there were 207 internet bloggers who have blogged about “gluten cross-reactive foods”, it must have been independently verified 207 times. Instead, it appears that this did not happen, and that these are examples of blatant regurgitation and misreporting without scrutiny.
Cross-reactivity in immunology has been defined as the ability for antibodies or self-reactive immune cells to recognize and bind different but similar antigens. So, if proteins in one substance are very similar to the proteins in another substance, there can be an immunological reaction to both. A good example of this is the Havein-like protein domains found in latex and bananas. People who develop sensitivity to latex are often also sensitive to bananas because they contain similar proteins. [4]
In the case of “gluten cross-reactive foods,” the argument is that antibodies or cells recognizing wheat or gluten can also recognize other foods, such as eggs, chocolate, or coffee.
3 | CELIAC DISEASE (CD) AND NON-CELIAC GLUTEN SENSITIVITY (NCGS)
About 1 in 133 people have Celiac disease, an autoimmune disease that damages the intestine. [5] The autoimmune reaction generates cells and antibodies which recognize gluten or gluten complexed with tissue transglutaminase (tTG).
The most common portion of the wheat gluten protein that is recognized is called α-gliadin. As a result of this, individuals with CD have cells which make anti-α-gliadin and anti-tTG antibodies (among others), and these are detectable using blood tests. CD is diagnosed with these blood tests along with a confirmatory intestinal biopsy.
Non-Celiac Gluten Sensitivity (NCGS) is different. In general those with NCGS test negative for these autoantibodies — there is currently no biomarker or blood test to diagnose NCGS. The paper discussed here specifically deals with antibodies specific to α-gliadin peptide. Therefore, this paper can potentially only apply to individuals with CD and NOT those with NCGS. You can’t have “gluten cross-reactive” antibodies without anti-gluten or anti-tTG antibodies to start with. Though the authors use the term “gluten sensitivity” in the paper discussed below, they are not referring to those with NCGS, and instead are using the term somewhat synonymously with CD.
4 | GLUTEN-ASSOCIATED CROSS REACTIVITY
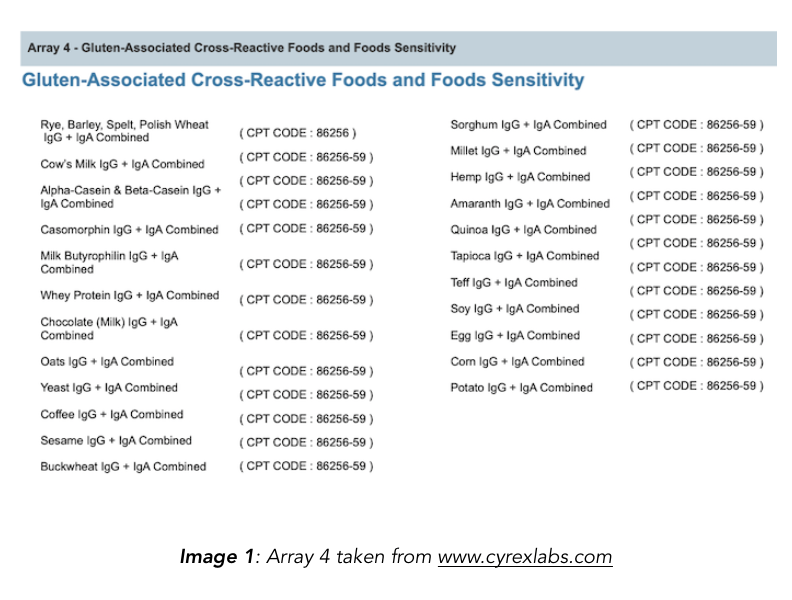
The “gluten-associated cross-reactive” foods list has been compiled from the Cyrex Labs Array 4. The Chief Scientific Officer of Cyrex, Aristo Vojdani, also used the specific foods listed in the array as antigens in a publication assessing their reactivity to α-gliadin antibodies (Vojdani & Tarash 2013).
Most of the articles on gluten cross-reactive foods list all 18 foods covered on the Cyrex panel, extrapolating to say that all are or can be cross-reactive to gluten. However, not all of these foods were determined to be gluten cross-reactive in their studies. For example, hemp and potatoes were never demonstrated to be cross-reactive, or have the potential for cross-reactivity.
In addition to these reporting errors, some individuals have added foods to this “gluten cross-reactive” list that doesn’t even show up in the conclusions of the original paper and have no additional citations, including sugar, “chemicals,” and GMO’s.
Further, the panel does not appear to assess reactivity of α-gliadin specific antibodies to other foods, but rather measures direct IgG antibody production against those foods.
5 | VOJDANI & TARASH 2013, CYREX LABS ARRAY 4
The following are the main highlights and conclusions of Vojdani & Tarash 2013 which deal with the “gluten cross-reactive foods” found on the Cyrex Labs Array 4.
RATIONALE
1. Some CD patients (~30%) continue to experience symptoms despite adopting a gluten-free diet (GFD). [Note: they did not study those with NCGS]
2. The authors hypothesized if continued symptoms despite the adoption of a GFD could be caused by cross-contamination with gluten-containing foods or with cross-reactivity between α-gliadin (the main immunogenic epitope of wheat in celiac disease).
3. This study measured the reactivity of α-gliadin antibodies (both polyclonal and monoclonal) against α-gliadin and other food antigens using ELISA and dot-blot.
METHODS
1. Purchased foods from the supermarket and processed them for study. The left side of [TABLE 1] shows foods that were used for antigen testing. Note that the foods used are identical to the foods contained in the Cyrex Array 4 which then show up on every “19 gluten cross-reactive foods list.”
2. Polyclonal rabbit anti-α-gliadin antibodies were made from rabbits injected with α-gliadin.
3. Highly specific monoclonal mouse anti-α-gliadin antibodies were purchased from a commercial source.
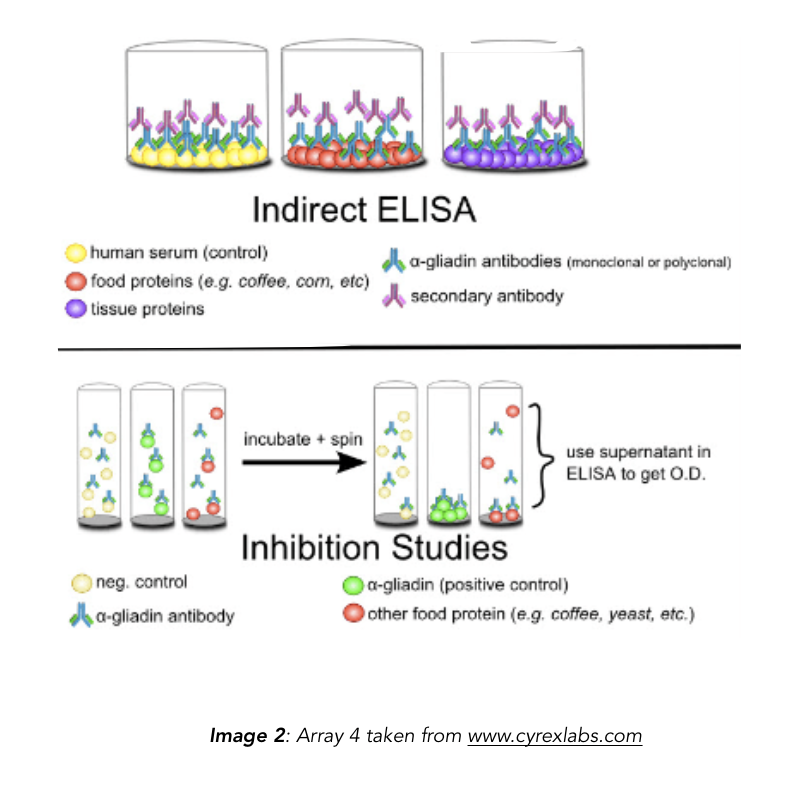
4. Used indirect ELISA and dot blot analysis to assess cross-reactivity of polyclonal and monoclonal anti-α-gliadin antibodies to food proteins.
RESULTS
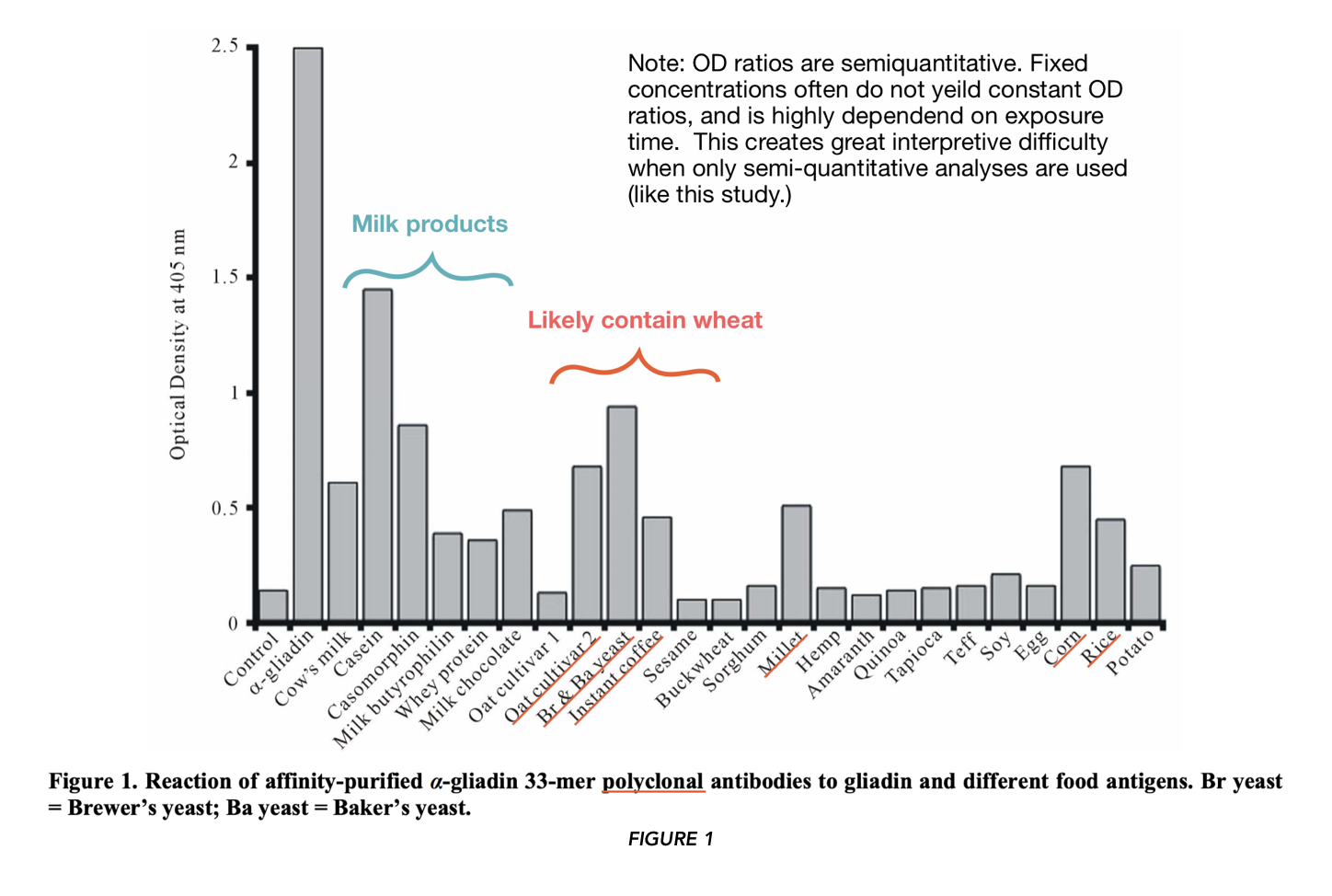
In FIGURE 1 and 2 (using polyclonal and monoclonal antibodies respectively), the authors asked whether certain food proteins would be recognized from antibodies specific for anti-α-gliadin. They found high binding against α-gliadin (positive control).
They also found binding “…against α– + β-casein, followed by yeast, casomorphin, oat cultivar #2, fresh corn, milk, millet, milk chocolate, instant coffee, rice, milk butyrophilin, and whey protein.” They did not find binding of oat cultivar #1, sesame, buckwheat, sorghum, hemp, amaranth, quinoa, tapioca, teff, soy, egg, and potatoes — foods commonly cited as “gluten cross-reactors.“ Note that only the mean for the assays (stated to be performed in quadruplicate) are reported, that “error bars” are not included, nor is there comment on whether the results were significantly different from the control.
By this standard, I could flip a coin, get tails 3/4 times, and conclude tails is 3 times as common as heads.
Scientific conclusions can only be trusted when tested to be true with statistical significance (typically p≤0.05).
RESULTS ABSORPTION/INHIBITION STUDIES
The authors asked: can food proteins displace binding of the α-gliadin-specific antibody to α-gliadin?
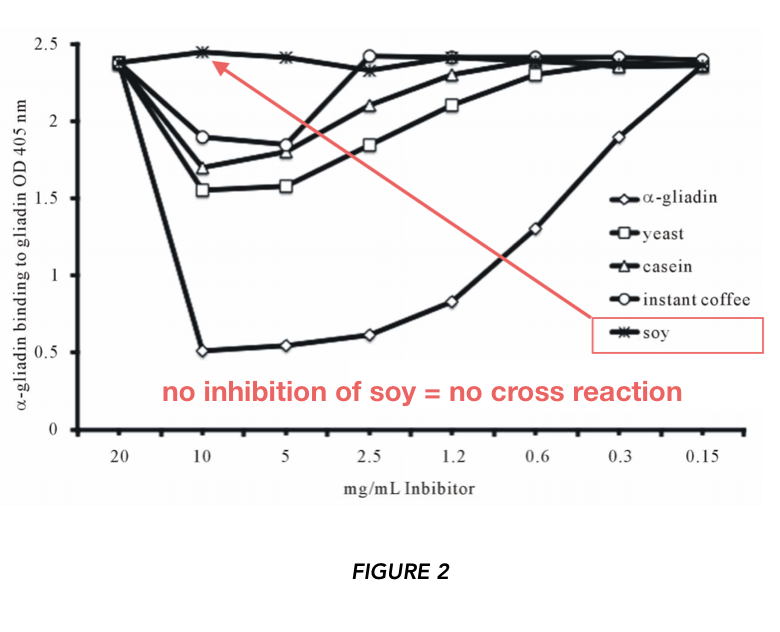
They used high (10mg/ml) and low (150 ug/ml) concentrations of the proteins. At the high concentration, α-gliadin inhibited anti-α-gliadin antibody binding to plate-bound α-gliadin at 79% (this is the positive control). At the low concentration, no displacement was observed. At higher concentrations, yeast, instant coffee, and casein appear to promote displacement of anti-α-gliadin antibodies to α-gliadin. They also report no inhibition from soy at any concentration.
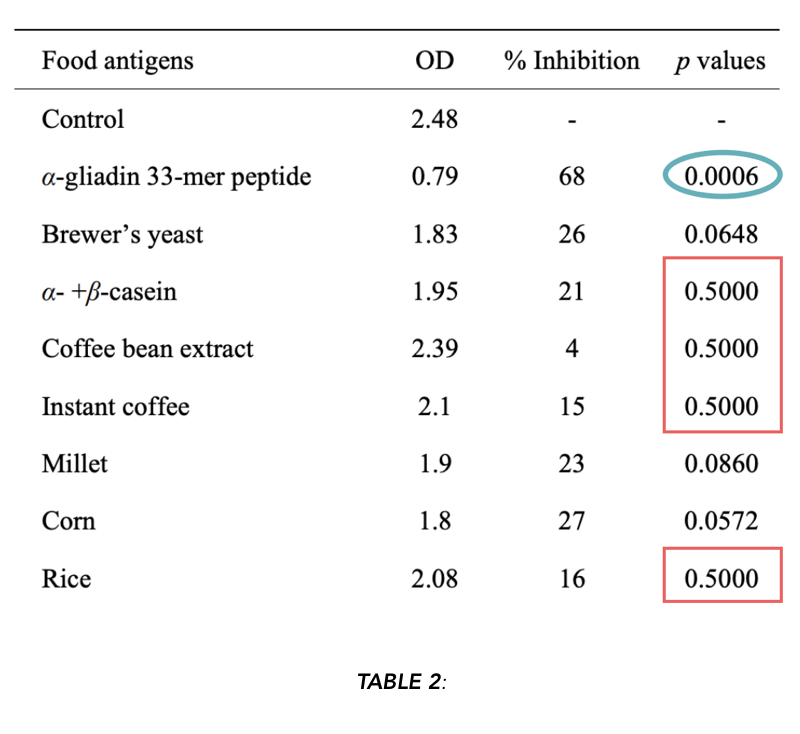
“The absorption with corn, yeast, millet, α– + β-casein, instant coffee and rice antigens inhibited the binding of α-gliadin 33-mer peptide to anti-α-gliadin 33-mer peptide by 27%, 26%, 23%, 21%, 16%, and 15%, respectively.”
STATISTICAL SIGNIFICANCE
While the authors report differences in the means, are they actually significant differences? First, we need to evaluate what the specific hypotheses for this particular experiment are.
1. Null hypothesis: (Food) antigen X does not inhibit α-gliadin antibody binding relative to control.
2. Alternate hypothesis: (Food) antigen X inhibits α-gliadin antibody binding relative to control
Biologists typically use a threshold value of p ≤ 0.05, which is considered to be statistically significant, and p ≥ 0.05 as non significant (this threshold value may change depending on the application and appropriateness for a given assay). A p ≤ 0.05 means that if we assume the null hypothesis (no effect) to be true, the chance of getting a result at least as large as what was observed, is equal to (or less than) 5%. Therefore, if we get a very small p-value (under 5%), we can reject the null hypothesis because the frequency of seeing such results under this hypothesis is so small that it’s incompatible. Meaning these results are unlikely to occur under the null hypothesis/model. The smaller the p-value, the less consistent the results are with the null hypothesis. Therefore, we might accept the alternative hypothesis, which is likely to be more compatible with the observed results.
Observing the “p-values” reported in Table 2:
Using the p ≤ 0.05 threshold, we can reject the null hypothesis for α-gliadin (outlined in blue, positive control). If we’re generous and we increase our threshold to p ≤ 0.1 (meaning the frequency of getting results at least this large under the null hypothesis is less than or equal to 10%), we can include yeast, millet, and corn.
p = 0.5 means that there is a 50% chance of getting results at least as large as what was observed under the null hypothesis of no effect. In other words, if we repeated this experiment several times, half of the time, we’d see differences at least this big, even if there was no actual effect.
This calls into question the interpretation of the results, because the results are so consistent with the null hypothesis of no effect. While the means of the assays (reported as a % inhibition) may have been reduced (indicating binding), there was apparently such high assay variability that it was not a significant difference from the control. Therefore, these results are consistent with the hypothesis of no effect, and consistent with the idea that the differences we see are due to chance.
Dot blot analyses (Fig 8 and Fig 9 of the paper, using polyclonal and monoclonal antibodies respectively) showed slight staining (possibly indicating antibody reactivity) against instant coffee, corn, yeast, milk, α– + β-casein, millet, rice, milk butyrophilin and oats.
6 | VOJDANI & TARASH 2013 CONCLUSIONS
No staining was observed with casomorphin, egg, pure coffee bean, cocoa, and sorghum. A note on the dot blot experiment: polyclonal antibodies (used in Fig. 1 and 8) are notorious for yielding high background (i.e. not real) signal. [6]
Secondary lactose (milk sugar) intolerance is common in people with CD. [7] It has also been demonstrated that about 50% of CD patients (who do not get better on a GFD alone) can have a specific mucosal response to cows milk, with casein being the likely culprit. [8]
Antibodies to milk proteins can be elevated for some people; these responses have not been demonstrated to be due to mimicry or cross-reactivity. [9] As Vojdani & Tarash 2013 cite, there is some computational data to suggest that these antibodies may cross-react, and some of their own data might also suggest that is the case. [10]
COFFEE
Instant coffee often contains gluten. Be aware of labels which state “may contain traces of gluten.”
OATS
Some oats contain gluten. We already knew this, since, though they are different plants, and oats do not contain gluten, they are processed together, and therefore are often contaminated with wheat/gluten/α-gliadin. If you do eat oats, you should make sure they are tested to be gluten-free; even being processed in an oat-only facility may not be enough. [11]
People with Celiac disease may also have T-cell specific reactions to the oat prolamine avenin.
Monoclonal antibodies against γ-gliadin (not α-gliadin antibodies like are used in this study) can react to oat avenin. [12]
However, avenin is not proline-rich like gliadin and therefore is able to be better degraded by GI tract enzymes, and this lessens potential immune reactions. Additionally, studies looking at immune activation in the small intestines of Celiac disease patients (following a GFD) failed to show histological consequences of immune activation following oat consumption. [13]
However, some studies do demonstrate oat-specific responses particularly in patients with complicated Celiac disease, although these are typically not large or randomized studies. [14] Moreover, the variety of the oat can make a difference, and this was reported in Vojdani & Trash 2013. [15]
YEAST
Vojdani & Tarash 2013 conclude that they “don’t know” whether the reaction they observed is a true cross-reaction or if the yeast used is contaminated with gluten. In these experiments, Brewer’s and Baker’s yeast appear to be combined when cross-reactivity was assessed.
CHOCOLATE
Reactivity was noticed with milk chocolate (see results from milk section). There was no reaction with pure cocoa or milk-free dark chocolate. Note that many milk chocolate products can be cross-contaminated with wheat, so it’s important to purchase chocolate that has been certified grain-free and gluten-free.
SOY
As indicated in Figure 3, there was no inhibition of anti-α-gliadin antibody binding when soy was added. However, the dot blots and other data are potentially suggestive. The inconsistency of reported results (i.e. which foods are “gluten cross-reactive?”) is concerning.
Note that milled soy flour may be contaminated by gluten (the linked pilot study found more than 2,900 ppm of gluten in one product). [16]
MILLET
Vojdani & Tarash 2013 conclude (based on Figures 1 & 2 and 4 & 5) that “millet may not be a good substitute for gluten-containing grains for some individuals” suggesting that this is due to “gluten cross-reactivity.” It is important to note that milled millet often contains contaminating gluten — even on those products listed as gluten-free, or “naturally gluten-free.” [17]
This makes millet often unsuitable due to contaminating gluten, and not necessarily due to cross-reactivity. The authors note a reference stating “amylase inhibitor from barley had significant homology with millet” however this does not appear to have any direct bearing on the antibodies specific for anti-α-gliadin used in this study.
CORN AND RICE
These are briefly discussed in the conclusions of Vojdani & Tarash 2013, only to state that “We also obtained fresh corn on the cob and various rice
grains and observed significant immune reactivity…” and make mention of a lipid transfer protein with ” high cross-reactivity rate with various grains and vegetables.”
The cited studies appear to be specific for allergic (IgE-mediated) type reactions. Other studies have suggested that corn might be a problem for some people with Celiac disease, and some zein moieties are computationally predicted to have sequence similarity to α-gliadin. [18]
However, CD-specific antibodies don’t appear to cross-react to corn zein. The rice/gluten cross-reactive study cited by Vojdani & Tarash 2013 is specific for IgE mediated responses, whose dominant epitopes are different than the epitopes recognized by anti-α-gliadin antibodies presented in this study. [19]
7 | DISCUSSION
Because extra-intestinal symptoms of CD have been reviewed in the scientific discourse [21], I will not be commenting on the portions of this paper discussing reactivity to tissue antigens.
8 | CONCLUSIONS
Patients on a gluten-free diet should adhere to a zero-tolerance poicy. This requires not only abstinence even from ‘gluten-free’ foods, but also
reading labels carefully, checking for the country of manufacturing origin, and on-label statements such as “produced in a factory that also processes wheat, gluten, and dairy”.
However, The Celiac Disease Center does not currently recognize Enterolabs or Cyrex stool tests for cross-reactivity (or for CD for that matter). [20] Simply, they are “not sensitive or specific enough” and just haven’t held water (yet) in the scientific arena.
9 | NOTE FROM THE AUTHOR
I would like to plead with the audience to take this post for what it is: a critical review of an article which has been misinterpreted and has spread throughout the blog-o-sphere like wildfire; it is intended to start a conversation.
It is not a personal attack on the authors of the study, Cyrex labs, practitioners who use Cyrex labs, nor is it an attack on The Paleo Mom, whose work I very much appreciate and admire. I am very much open to continued discussion on the methods, conclusions, and results of the particular paper discussed here, as well as links to any scientific and peer-reviewed articles. I also don’t want this post to detract from legitimate arguments concerning the avoidance of some of the foods on the Cyrex Array 4 list. I believe it is true that “gluten-free isn’t always enough” and this is true for those with CD as well as NCGS.
There are many reasons to avoid other foods than gluten, and it is relatively easy (and cheap) to determine other foods you are sensitive to using a food journal and food rotation diet. I myself am
diagnosed with NCGS, and have found symptom relief from several autoimmune and autoinflammatory diseases with a gluten-free diet and also with the avoidance of sugar, non-gluten-containing grains, alcohol, and other foods – some of which are on the Cyrex Array 4.
My only purpose is to highlight the importance of being rigorous with our own research and reporting within the ancestral health community and to highlight that the rationale for the avoidance of some foods may have arrived through the inflated interpretation of inconclusive results.
Scientific research is only beginning to reveal the intricacies of “leaky gut” and how it may promote the development of or exacerbate autoimmunity — and only time, clever questions, and intelligently designed experiments will yield fruitful results to push forward clinical and scientific dogma.
1. Ballantyne, Sarah. (March 13, 2013). Gluten Cross-Reactivity: How your body can still think you’re eating gluten even after giving it up. Retrieved from https://www.thepaleomom.com/gluten-cross-reactivity-update-how-your-body-can-still-think-youre-eating-gluten-even-after-giving-it-up/
2. Myers, Amy. (N.D.) Are You Not Healing Because Your Body Thinks Coffee, Chocolate & Cheese Are Gluten? Retrieved from https://www.mindbodygreen.com/0-7875/are-you-not-healing-because-your-body-thinks-coffee-chocolate-cheese-are-gluten.html
3. Perlmutter, David. (N.D) Gluten Associated Cross Reactive Foods. C https://www.drperlmutter.com/eat/foods-that-cross-react-with-gluten/
4. Frank SA. Immunology and Evolution of Infectious Disease. Princeton (NJ): Princeton University Press; 2002. Chapter 4, Specificity and Cross-Reactivity. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2396/
5. Adams, Jefferson. (March 30, 2009). Celiac Disease Statistics. Retrieved from https://www.celiac.com/articles.html/celiac-disease-statistics-r1147/
6. Abcam. (N.D) What are polyclonal, monoclonal and recombinant antibodies? Retrieved from https://www.abcam.com/protocols/a-comparison-between-polyclonal-and-monoclonal
7. Plotkin, G. R., & Isselbacher, K. J. (1964). Secondary Disaccharidase Deficiency in Adult Celiac Disease (Nontropical Sprue) and Other Malabsorption States. New England Journal of Medicine, 271(20), 1033–1037. doi:10.1056/nejm196411122712003
8. Kristjánsson, G., Venge, P., & Hällgren, R. (2007). Mucosal reactivity to cow’s milk protein in coeliac disease. Clinical & Experimental Immunology, 147(3), 449–455. doi:10.1111/j.1365-2249.2007.03298.x
9. Scott H, Fausa O, Ek J, Brandtzaeg P. (1984). Immune response patterns in coeliac disease. Serum antibodies to dietary antigens measured by an enzyme linked immunosorbent assay (ELISA). Clin Exp Immunol. Jul;57(1):25-32.
10. Darewicz, M., Dziuba, J., & Minkiewicz, P. (2007). Computational Characterisation and Identification of Peptides for in silico Detection of Potentially Celiac-Toxic Proteins. Food Science and Technology International, 13(2), 125–133. doi:10.1177/1082013207077954
11. Thompson, T. (2004). Gluten Contamination of Commercial Oat Products in the United States. New England Journal of Medicine, 351(19), 2021–2022. doi:10.1056/nejm200411043511924
12. Mitea, C., Kooy-Winkelaar, Y., van Veelen, P., de Ru, A., Drijfhout, J. W., Koning, F., & Dekking, L. (2008). Fine specificity of monoclonal antibodies against celiac disease–inducing peptides in the gluteome. The American Journal of Clinical Nutrition, 88(4), 1057–1066. doi:10.1093/ajcn/88.4.1057
13. Srinivasan, U., Jones, E., Carolan, J., & Feighery, C. (2006). Immunohistochemical analysis of coeliac mucosa following ingestion of oats. Clinical and Experimental Immunology, 144(2), 197–203. doi:10.1111/j.1365-2249.2006.03052.x
14. Arentz-Hansen, H., Fleckenstein, B., Molberg, Ø., Scott, H., Koning, F., Jung, G., … Sollid, L. M. (2004). The Molecular Basis for Oat Intolerance in Patients with Celiac Disease. PLoS Medicine, 1(1), e1. doi:10.1371/journal.pmed.0010001
15. Comino, I., Real, A., de Lorenzo, L., Cornell, H., Lopez-Casado, M. A., Barro, F., … Sousa, C. (2011). Diversity in oat potential immunogenicity: basis for the selection of oat varieties with no toxicity in coeliac disease. Gut, 60(7), 915–922. doi:10.1136/gut.2010.225268
16. Thompson, T., Lee, A. R., & Grace, T. (2010). Gluten Contamination of Grains, Seeds, and Flours in the United States: A Pilot Study. Journal of the American Dietetic Association, 110(6), 937–940. doi:10.1016/j.jada.2010.03.014
17. Thompson, T., Lee, A. R., & Grace, T. (2010). Gluten Contamination of Grains, Seeds, and Flours in the United States: A Pilot Study. Journal of the American Dietetic Association, 110(6), 937–940. doi:10.1016/j.jada.2010.03.014
18. Cabrera-Chávez, F., Iametti, S., Miriani, M., de la Barca, A. M. C., Mamone, G., & Bonomi, F. (2012). Maize Prolamins Resistant to Peptic-tryptic Digestion Maintain Immune-recognition by IgA from Some Celiac Disease Patients. Plant Foods for Human Nutrition, 67(1), 24–30. doi:10.1007/s11130-012-0274-4
19. Denery-Papini, S., Bodinier, M., Larré, C., Brossard, C., Pineau, F., Triballeau, S., … Moneret-Vautrin, D.-A. (2012). Allergy to deamidated gluten in patients tolerant to wheat: specific epitopes linked to deamidation. Allergy, 67(8), 1023–1032. doi:10.1111/j.1398-9995.2012.02860.x
20. Celiac Disease Center. (2013) Frequently Asked Questions. Retrieved from http://www.cureceliacdisease.org/faq/why-dont-you-recognize-tests-stool-tests-or-otherwise-for-gluten-sensitivity-that-are-currently-available-through-companies-like-enterolab-or-cyrex/
21. Hadjivassiliou, M., Williamson, C. A., & Woodroofe, N. (2004). The immunology of gluten sensitivity: beyond the gut. Trends in Immunology, 25(11), 578–582. doi:10.1016/j.it.2004.08.011
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Exogenous Ketones, Ketone Esters and Ketone Salts
Exogenous Ketones, Ketone Esters and Ketone Salts