Should Xanthan Gum be allowed in Grain-Free, Paleo, and KETO Certified Standards?
Should Xanthan Gum be allowed in Grain-Free, Paleo, and KETO Certified Standards?
Abstract
The gummy, purified secretion of the bacteria Xanthomonas Campestris, is best known by its completely trivial name ‘xanthan gum.’ Xanthan gum is a common food additive that is prevalent in many grain-free and gluten-free foods. While xanthan gum is generally accepted as safe (GRAS) for use in food additives without any specific quantity limitations, it is still the subject of controversy, misconception, and cause for confusion. Here we consider the production, properties, and place in the diet of xanthan gum to determine its applicability within the Grain-Free, Paleo, and KETO Certified programs.
KEYWORDS
Xanthan Gum, polysaccharides, dietary fiber, prebiotics
Xanthan gum is an ingredient commonly found in packaged foods, yet few know what it is or why it’s there. Its obscurity and complicated name have lead many to assume that it’s a harmful chemical, and several influential health leaders have discouraged its consumption. [1, 2].
As a third-party certification organization, The Paleo Foundation is trusted and tasked with making decisions about the place of such food ingredients within the framework of the Grain-Free, Paleo, and KETO Certification program standards. We do not make these decisions without careful consideration of available data, benefits, and uncertainties.
In this research report, we will examine the research that is available on the compound to help clear up many of the misconceptions regarding xanthan gum in the diet, as well as offer our final decision on the applicability of xanthan gum in the standards.
Xanthan gum is an extracellular polysaccharide or exopolysaccharide (EPS) that is secreted by the bacteria Xanthomonas campestris, first discovered at the Northern Regional Research Laboratories (NRRL) in the 1950s [3].
EPS are naturally produced by many microorganisms, including bacteria, yeasts, and fungi. These organisms synthesize EPS and excrete them out of the cell, as they serve to protect them from desiccation, microphage attack, antibiotics, and toxic compounds.
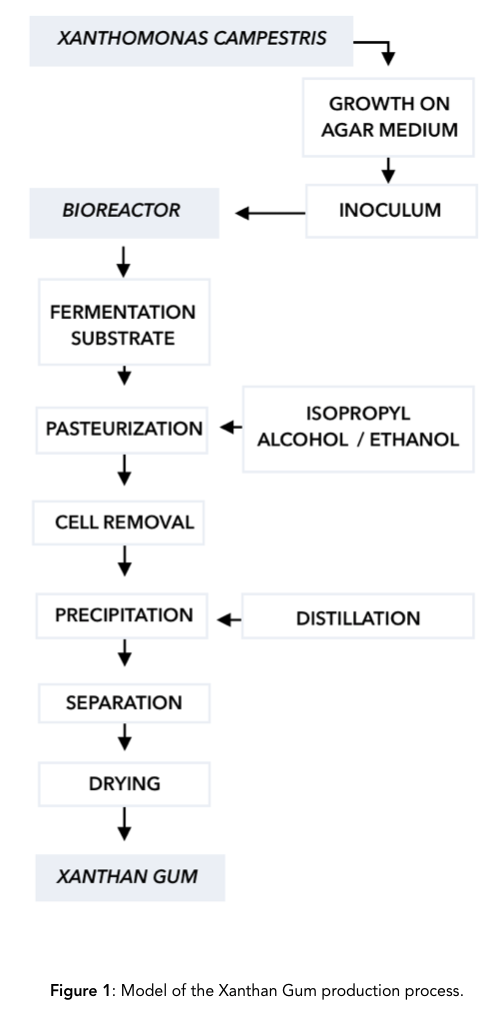
The medium that Xanthomonas campestris is most frequently grown on is agar, a jelly-like substance obtained from red algae for 18±20 h at 25°C,[4] and the substrate most often given to the bacteria to produce xanthan gum are glucose and sucrose. After the bacteria feed on the substrates, they secrete a polysaccharide compound that is dried up to form a powder, and sold as xanthan gum. [Figure 1]
Extensive research was carried out in several industrial laboratories during the 1960s because of the properties that would allow it to supplement other natural, water-soluble gums, and was in commercial production by 1964. [3]
Xanthan gum’s main polysaccharide chain consists of b-D-glucose units linked at the 1 and 4 positions, a chemical structure is identical to that of cellulose. The molecular weight distribution of xanthan gum can range from 2 x 10⁶ to 20 x 10⁶ Da, depending on the association between the chains and the variations of the fermentation conditions used during production.[5]
As a result of the high molecular weight, complex branching, and interaction with other polymers, solutions of xanthan gum tend to be highly viscous. And, due to its viscosity, xanthan gum has practical use in a wide variety of foods for its stabilizing, thickening, particle-suspending, and rheological properties.
FUNCTIONALITY IN FOOD
In gluten-free baking, xanthan gum contributes to the smoothness, “airiness”, and air retention of cakes, muffins, biscuits, and bread mixes. Baked goods that incorporate xanthan gum also have increased volume and moisture, less crumbling, and enhanced resistance to shipping damage. [6]
In wet-prepared batters, xanthan gum reduces flour sedimentation, improves gas retention and freeze-thaw stability, while improving spread control, and volume. Xanthan gum also improves volume, texture, and moisture retention in refrigerated doughs, gluten-free breads, and pasta. [6]
Dressings formulated with xanthan gum also have excellent long-term stability and a relatively constant viscosity over a wide temperature range. And, due to the pseudoplastic properties of xanthan gum, dressings incorporating xanthan gum pour easily but cling well to foods. [6]
In frozen foods, xanthan gives stability and consistent viscosity during freeze-thaw cycles and heating. In some applications such as in ice creams, xanthan gum prevents ice crystal formation and improves the texture and mouthfeel of the end product as well. [7]
In dry mixes, xanthan gum is used as a grain-free, gluten-free thickener for desserts, dips, soups, milkshakes, sauces, gravies, and beverages. In fact, dry mixes for instant thickening of beverages such as water, fruit juice, green tea, or milk are sold for use for individuals suffering from dysphagia (difficulty swallowing). [8]
4 | DIETARY CONSIDERATIONS
Xanthan gum is recognized as a food additive under the provisions of the US Food and Drug Administration (FDA) regulations (21 CFT 172.695) for use as a stabilizer, thickener, or emulsifier. [9] Xanthan gum is designated by the European Union as E415 with a non-specified acceptable daily intake (ADI). [10]
TOXICITY
In 2017, a scientific panel from the European Food Safety Authority (EFSA), which is reputably very conservative about the safety of food ingredients, conducted a safety review of xanthan gum and found that it caused no adverse events (even when consumed at high doses), found no evidence of it being a genotoxin when consumed in the long term, and that xanthan gum was likely of no concern to the general public as a food additive [11].
In one human study where xanthan gum was repeatedly consumed at high doses (from 10.4 to 2.9 g of xanthan gum per day / 149–184 mg/kg body weight), xanthan gum was found to have no adverse dietary or physiological effects.
12.9 g of xanthan gum per day / 149–184 mg/kg body weight), xanthan gum was found to have no adverse dietary or physiological effects. However, stool bulking, reduced GI transit time, a moderate (10%) reduction in serum cholesterol (p < 0.05), and a significant increase in fecal bile acid concentrations (p < 0.05), and significant increases in short-chain fatty acid production (SCFAs) (p < 0.05), were observed. [12] Of note, these effects are generally regarded as being incredibly desirable from a health perspective.
SHORT-CHAIN FATTY ACIDS
SCFAs are a subset of fatty acids that are produced by the gut microbiota during the fermentation of partially and non-digestible polysaccharides and are a major player in the maintenance of gut and immune homeostasis.
SCFAs, particularly butyrate, are key promoters of colonic health and integrity. Butyrate is the major and preferred metabolic substrate for colonic epithelia, providing at least 60–70% of their energy requirements, which are key to the maintenance of large intestinal function.[13]
Another study found that among other gums such as gum arabic and guar gum, xanthan gum yielded a large amount of total SCFAs, including acetic, propionic, and butyric acids compared to other gums [14].
Other studies on the in vitro degradation and the in vivo digestibility of xanthan gum performed in animals and humans have demonstrated that xanthan gum would not be absorbed intact, and would not be metabolized by enzymes in the gastrointestinal tract.[11]
However, it would be partially fermented during its passage through the large intestine by the action of the intestinal tract microbiota, making xanthan gum a candidate for prebiotic status. [11]
PREBIOTICS AND DIETARY FIBERS
Today, prebiotics and dietary fiber are well-studied for their role in gut health maintenance, colitis prevention, cancer inhibition, immunopotentiation, improvements in cholesterol, reduction of cardiovascular disease, prevention of obesity, and constipation. [15]
Prebiotics are generally defined as nondigestible polysaccharides and oligosaccharides, which show resistance to gastric acidity, hydrolysis by mammalian enzymes and gastrointestinal absorption, and selective stimulation of the growth and/or activity of beneficial intestinal bacteria that exert antagonism towards pathogenic bacteria, limiting their proliferation. [16]
Current, and revised definitions of dietary fibers include not only nondigestible plant polysaccharides but also microbial exopolysaccharides (EPS). These include xanthan gum as well as other EPS like gellan gum, pullulan, and dextran. Foods containing EPS fibers are mostly known for their ability to prevent or relieve constipation, reduce cholesterol, and enhance host immune system function. [17]
Xanthan gum is a 100% dietary fiber and only 25 grams are needed to meet daily values. Nondigestible, non-starch polysaccharides are known to confer other health benefits, such as helping to maintain a healthy weight and lowering the risk of coronary heart disease, diabetes, obesity, and some forms of cancer. In short, xanthan gum is a dietary fiber and polysaccharide which resists hydrolysis, which confers a benefit to the human host.
5 | HEALTH BENEFITS OF XANTHAN GUM
Lowers cholesterol: A study found that men who consumed ten times the recommended daily amount of xanthan gum for 23 days experienced a 10% decrease in cholesterol [25].
Satiety: Xanthan gum may promote satiety and delay stomach emptying, thereby helping individuals feel fuller, longer [26, 27].
Anti-cancer: An in vivo study on melanoma found that xanthan gum significantly slowed the growth of cancerous cells via immunostimulation, increasing survival expectancy [ 28 ].
Blood Sugar: In a 12-week study of subjects with diabetes, administration of 12 grams of xanthan gum was found to decrease blood sugar levels during fasted states, and 2 hours post-prandially without digestive symptoms [27]. Another randomized trial found that
subjects given juice mixtures with xanthan gum experienced the largest reduction in blood sugar compared to the control [29]. Another study found that it suppressed the blood sugar spike that typically occurs 30 minutes after the consumption of rice [30].
Improved Bowel Movement: Xanthan gum increases the movement of water into the intestines to create a softer, bulkier stool that’s easier to pass. In a study of 18 volunteers, it was found that it significantly increased stool frequency and amount [ 31 ].
Dry Mouth: Xanthan gum may be used as a substitute for saliva in individuals suffering from dry mouth. Double-blind RCT of 33 patients with Sjogren’s syndrome used xanthan gum-based saliva substitutes, which were found to improve dry-mouth [30]. A randomized trial of 65 patients on fluid-restricted diets showed saliva substitutes containing xanthan gum could significantly improve symptoms of extreme thirst and dry mouth [32].
Dysphagia: Dysphagia is a disorder characterized by difficulty swallowing which is common in certain neurological disorders, and post-stroke [33]. Used as a food and liquid thickener, it improved the safety of foods and provided therapeutic relief for 120 patients with dysphagia [33], which was found to be superior in reducing adverse events such as choking [34].
Tooth Decay: A single-blinded, randomized controlled trial found that 16 subjects randomized for acidic fruit drinks mixed with xanthan gum experienced significantly less enamel loss in comparison to controls [35].
6 | ADVERSE EFFECTS OF XANTHAN GUM
DIGESTIVE DISCOMFORT
Flatulence: In human trials, xanthan gum was found to cause some digestive discomfort, including laxative effects, bloating, and excess flatulence. However, over the course of 10 days, 16 of 18 subjects could metabolize xanthan gum compared to 12 at the beginning, indicating an improvement in xanthan gum metabolism over time. [31].
This study also found a significant increase in the production of hydrogen and SCFAs, indicative of bacterial adaptation in the presence of a Xanthan Gum substrate during the course of the trial. Further, flatus and digestive discomfort decreased over the course of the 10-day trial, as the substrate was better assimilated by colonic bacteria.
NECROTIZING ENTEROCOLITIS
Necrotizing enterocolitis (NEC) is a condition, typically occurring in preterm infants, where a part of the intestinal tissue dies. Of those affected, nearly one in four suffer mortality. Before 2011, there were reports that the product Simply Thick, which had xanthan gum as an active ingredient in infant formula, was increasing rates of the condition. In 2011, the FDA issued a warning that Simply Thick could cause necrotizing enterocolitis in infants and discouraged its use in food products for infants [37– 39].
While this was clearly an appropriate decision for the health agency, it does not implicate xanthan gum as the cause of necrotizing enterocolitis, as the exposure could have also been any of the other inactive ingredients within Simply Thick. Furthermore, more recent data suggests that chorioamnionitis, or intra-amniotic infection of the fetal membranes due to a bacterial infection, is the most important risk factor for the vast majority of NEC cases. [40]
This could explain why the FDA did not put out a statement suggesting that xanthan gum causes necrotizing enterocolitis. The serious adverse events that were reported to the FDA all indicated that Simply Thick was the exposure. Therefore, the argument that xanthan gum is the sole cause of necrotizing enterocolitis is not robust.
In fact, in clinical studies involving infants, xanthan gum in infant formula was found to be well-tolerated, did not influence minerals (Ca, P, Mg), fat and nitrogen balance, and did not affect growth characteristics up to a concentration of 1,500 mg/L (232 mg/kg BW per day).
These results were supported by the outcome of the post-marketing surveillance of a formula containing xanthan gum at a concentration of approximately 750 mg/L. [41]
However, even if xanthan gum did cause necrotizing enterocolitis in premature infants, it does not suggest that the food additive would lead to the same outcomes in adults. Many food products that adults are able to consume are not appropriate to give to infants, such as honey. [42]
In essence, it would be short-sighted to assume that xanthan gum is unsafe for the general population because a product that contained it as an active ingredient was linked to necrotizing enterocolitis in infants.
7 | DISCUSSION
It is clear that xanthan gum is not as harmful as it is often believed to be, and two health agencies have determined it to be safe, even with long-term consumption and appreciably large doses. Further, as a dietary fiber with prebiotic activity, xanthan gum deserves a more welcoming attitude than it is currently afforded.
8 | CONCLUSION
From our assessment of the existing arguments and the evidence, The Paleo Foundation will support the inclusion of xanthan gum in Grain-Free, Certified Paleo, and Keto Certified program standards.
Further, it is likely that xanthan gum as a functional food ingredient will provide dietary fiber in many products, and may mitigate some of the potential adverse health effects associated with the long-term adoption of a low-fiber, ketogenic diet.
Despite the literature that clearly supports the addition of xanthan gum in the diet, some opposition is expected to arise from those in the community who believe xanthan gum is harmful. We believe that addressing these myths via education efforts is vital in promoting a diet that is based on evidence-based principles, rather than beliefs couched in distorted evidence and misinformation, perpetuated by misplaced fear.
9 | CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
1. Ballantyne S. 5 “Paleo” Things That Aren’t. In: The Paleo Mom [Internet]. 16 Jan 2017 [cited 3 Dec 2019]. Available: https://www.thepaleomom.com/5-paleo-things-arent/
2. Ballantyne S. Is It Paleo? Guar Gum, Xanthan Gum and Lecithin, Oh My! ~ The Paleo Mom. In: The Paleo Mom [Internet]. 2014 [cited 3 Dec 2019]. Available: https://www.thepaleomom.com/is-it-paleo-guar-gum-xanthan-gum-and-lecithin-oh-my/
3. Garcı́a-Ochoa, F., Santos, V. ., Casas, J. ., & Gómez, E. (2000). Xanthan gum: production, recovery, and properties. Biotechnology Advances, 18(7), 549–579. doi:10.1016/s0734-9750(00)00050-1
4. Cadmus, M. C., Rogovin, S. P., Burton, K. A., Pittsley, J. E., Knutson, C. A., & Jeanes, A. (1976). Colonial variation in Xanthomonas campestris NRRL B-1459 and characterization of the polysaccharide from a variant strain. Canadian Journal of Microbiology, 22(7), 942–948. doi:10.1139/m76-136
5. Fink, J. K. (2013). Thickeners. Hydraulic Fracturing Chemicals and Fluids Technology, 35–57. doi:10.1016/b978-0-12-411491-3.00003-0
6. Sworn, G. (2009). Xanthan gum. Handbook of Hydrocolloids, 186–203. doi:10.1533/9781845695873.186
7. Fernández, P. P., Martino, M. N., Zaritzky, N. E., Guignon, B., & Sanz, P. D. (2007). Effects of locust bean, xanthan and guar gums on the ice crystals of a sucrose solution frozen at high pressure. Food Hydrocolloids, 21(4), 507–515. doi:10.1016/j.foodhyd.2006.05.010
8. Rofes, L., Arreola, V., Mukherjee, R., Swanson, J., & Clavé, P. (2014). The effects of a xanthan gum-based thickener on the swallowing function of patients with dysphagia. Alimentary Pharmacology & Therapeutics, 39(10), 1169–1179. doi:10.1111/apt.12696
9. 7 CFR § 205.605 – Nonagricultural (nonorganic) substances allowed as ingredients in or on processed products labeled as “organic” or “made with organic (specified ingredients or food group(s)).” In: LII / Legal Information Institute [Internet]. [cited 29 Nov 2019]. Available: https://www.law.cornell.edu/cfr/text/7/205.605
10. Mortensen A, Aguilar F, Crebelli R, Domenico AD, Frutos MJ, Galtier P, et al. Re-evaluation of xanthan gum (E 415) as a food additive. EFSA J. 2017;15: e04909. doi:10.2903/j.efsa.2017.4909
11. Mortensen A, Aguilar F, Crebelli R, Domenico AD, Frutos MJ, Galtier P, et al. Re-evaluation of xanthan gum (E 415) as a food additive. EFSA J. 2017;15: e04909. doi:10.2903/j.efsa.2017.4909
12. Eastwood, M. A., Brydon, W. G., & Anderson, D. M. W. (1987). The dietary effects of xanthan gum in man. Food Additives and Contaminants, 4(1), 17–26. doi:10.1080/02652038709373610
13. Suzuki, T., Yoshida, S., & Hara, H. (2008). Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. British Journal of Nutrition, 100(02). doi:10.1017/s0007114508888733
14. Adiotomre, J., Eastwood, M. A., Edwards, C. A., & Brydon, W. G. (1990). Dietary fiber: in vitro methods that anticipate nutrition and metabolic activity in humans. The American Journal of Clinical Nutrition, 52(1), 128–134. doi:10.1093/ajcn/52.1.128
15. Vidanarachchi, J. K., Iji, P. A., Mikkelsen, L. L., Sims, I., & Choct, M. (2009). Isolation and characterization of water-soluble prebiotic compounds from Australian and New Zealand plants. Carbohydrate Polymers, 77(3), 670–676. doi:10.1016/j.carbpol.2009.02.009
16. Varzakas, T., Kandylis, P., Dimitrellou, D., Salamoura, C., Zakynthinos, G., & Proestos, C. (2018). Innovative and fortified food: Probiotics, prebiotics, GMOs, and superfood. Preparation and Processing of Religious and Cultural Foods, 67–129. doi:10.1016/b978-0-08-101892-7.00006-7
17. Nampoothiri, K. M., Beena, D. J., Vasanthakumari, D. S., & Ismail, B. (2017). Health Benefits of Exopolysaccharides in Fermented Foods. Fermented Foods in Health and Disease Prevention, 49–62. doi:10.1016/b978-0-12-802309-9.00003-0
18. Xanthan Gum 1 tbsp Nutrition Facts & Calories. [Internet]. N/A [cited 12 Dec 2019]. SELFNutritionData. Available: https://nutritiondata.self.com/facts/custom/1737252/2
19. Mann, J. I., & Cummings, J. H. (2009). Possible implications for health of the different definitions of dietary fibre. Nutrition, Metabolism and Cardiovascular Diseases, 19(3), 226–229. doi:10.1016/j.numecd.2009.02.002
20. Luo, J., Liu, J., Ke, C., Qiao, D., Ye, H., Sun, Y., & Zeng, X. (2009). Optimization of medium composition for the production of exopolysaccharides from Phellinus baumii Pilát in submerged culture and the immuno-stimulating activity of exopolysaccharides. Carbohydrate Polymers, 78(3), 409–415. doi:10.1016/j.carbpol.2009.04.038
21. Ramberg, J. E., Nelson, E. D., & Sinnott, R. A. (2010). Immunomodulatory dietary polysaccharides: a systematic review of the literature. Nutrition Journal, 9(1). doi:10.1186/1475-2891-9-54
22. Lin, M.-H., Yang, Y.-L., Chen, Y.-P., Hua, K.-F., Lu, C.-P., Sheu, F., … Wu, S.-H. (2011). A Novel Exopolysaccharide from the Biofilm ofThermus aquaticusYT-1 Induces the Immune Response through Toll-like Receptor 2. Journal of Biological Chemistry, 286(20), 17736–17745. doi:10.1074/jbc.m110.200113
23. Zhang, M., Cheung, P. C. K., & Zhang, L. (2001). Evaluation of Mushroom Dietary Fiber (Nonstarch Polysaccharides) from Sclerotia ofPleurotus tuber-regium(Fries) Singer as a Potential Antitumor Agent. Journal of Agricultural and Food Chemistry, 49(10), 5059–5062. doi:10.1021/jf010228l
24. He, P., Geng, L., Mao, D., & Xu, C. (2012). Production, characterization and antioxidant activity of exopolysaccharides from submerged culture of Morchella crassipes. Bioprocess and Biosystems Engineering, 35(8), 1325–1332. doi:10.1007/s00449-012-0720-6
25. Eastwood, M. A., Brydon, W. G., & Anderson, D. M. W. (1987). The dietary effects of xanthan gum in man. Food Additives and Contaminants, 4(1), 17–26. doi:10.1080/02652038709373610
26. Fabek, H., Messerschmidt, S., Brulport, V., & Goff, H. D. (2014). The effect of in vitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion. Food Hydrocolloids, 35, 718–726. doi:10.1016/j.foodhyd.2013.08.007
27. Osilesi, O., Trout, D. L., Glover, E. E., Harper, S. M., Koh, E. T., Behall, K. M., … Tartt, J. (1985). Use of xanthan gum in dietary management of diabetes mellitus. The American Journal of Clinical Nutrition, 42(4), 597–603. doi:10.1093/ajcn/42.4.597
28. Takeuchi, A., Kamiryou, Y., Yamada, H., Eto, M., Shibata, K., Haruna, K., … Yoshikai, Y. (2009). Oral administration of xanthan gum enhances antitumor activity through Toll-like receptor 4. International Immunopharmacology, 9(13-14), 1562–1567. doi:10.1016/j.intimp.2009.09.012
29. Paquin, J., Bédard, A., Lemieux, S., Tajchakavit, S., & Turgeon, S. L. (2013). Effects of juices enriched with xanthan and β-glucan on the glycemic response and satiety of healthy men. Applied Physiology, Nutrition, and Metabolism, 38(4), 410–414. doi:10.1139/apnm-2012-0207
30. Van der Reijden, W. A., van der Kwaak, H., Vissink, A., Veerman, E. C. I., & Amerongen, A. V. N. (1996). Treatment of xerostomia with polymer-based saliva substitutes in patients with Sjögren’s syndrome. Arthritis & Rheumatism, 39(1), 57–63. doi:10.1002/art.1780390108
31. Daly, J., Tomlin, J., & Read, N. W. (1993). The effect of feeding xanthan gum on colonic function in man: correlation with in vitro determinants of bacterial breakdown. British Journal of Nutrition, 69(03), 897. doi:10.1079/bjn19930089
32. Bots, C. P., Brand, H. S., Veerman, E. C. I., Korevaar, J. C., Valentijn-Benz, M., Bezemer, P. D., … Nieuw Amerongen, A. V. (2005). Chewing gum and a saliva substitute alleviate thirst and xerostomia in patients on haemodialysis. Nephrology Dialysis Transplantation, 20(3), 578–584. doi:10.1093/ndt/gfh675
33. Rofes, L., Arreola, V., Mukherjee, R., Swanson, J., & Clavé, P. (2014). The effects of a xanthan gum-based thickener on the swallowing function of patients with dysphagia. Alimentary Pharmacology & Therapeutics, 39(10), 1169–1179. doi:10.1111/apt.12696
34. Vilardell, N., Rofes, L., Arreola, V., Speyer, R., & Clavé, P. (2015). A Comparative Study Between Modified Starch and Xanthan Gum Thickeners in Post-Stroke Oropharyngeal Dysphagia. Dysphagia, 31(2), 169–179. doi:10.1007/s00455-015-9672-8
35. West, N. X., Hughes, J. A., Parker, D., Weaver, L. J., Moohan, M., De’Ath, J., & Addy, M. (2004). Modification of soft drinks with xanthan gum to minimise erosion: a study in situ. British Dental Journal, 196(8), 478–481. doi:10.1038/sj.bdj.4811186
36. Rich, B. S., & Dolgin, S. E. (2017). Necrotizing Enterocolitis. Pediatrics in Review, 38(12), 552–559. doi:10.1542/pir.2017-0002
37. Beal, J., Silverman, B., Bellant, J., Young, T. E., & Klontz, K. (2012). Late Onset Necrotizing Enterocolitis in Infants following Use of a Xanthan Gum-Containing Thickening Agent. The Journal of Pediatrics, 161(2), 354–356. doi:10.1016/j.jpeds.2012.03.054
38. SimplyThick Warning. [cited 3 Dec 2019]. Available: https://www.simplythick.com/Safety
39. Louis CS. Warning Too Late for Some Babies. In: Well [Internet]. 4 Feb 2013 [cited 3 Dec 2019]. Available: https://well.blogs.nytimes.com/2013/02/04/warning-too-late-for-some-babies/
40. Coggins, S. A., Wynn, J. L., & Weitkamp, J.-H. (2015). Infectious Causes of Necrotizing Enterocolitis. Clinics in Perinatology, 42(1), 133–154. doi:10.1016/j.clp.2014.10.012
41. Mortensen A, Aguilar F, Crebelli R, Domenico AD, Frutos MJ, Galtier P, et al. Re-evaluation of xanthan gum (E 415) as a food additive. EFSA J. 2017;15: e04909. doi:10.2903/j.efsa.2017.4909
42. Abdulla, C. O., Ayubi, A., Zulfiquer, F., Santhanam, G., Ahmed, M. A., & Deeb, J. (2012). Infant botulism following honey ingestion. BMJ case reports, 2012, bcr1120115153. doi:10.1136/bcr.11.2011.5153
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Is Grain-Free the New Gluten-Free for Autoimmune Disease?
Is Grain-Free the New Gluten-Free for Autoimmune Disease?
 Ketosis and Carbohydrate Consumption
Ketosis and Carbohydrate Consumption