Propylene Glycol Alginate: All You Need to Know
Propylene Glycol Alginate Explained for Keto
Propylene glycol alginate (PGA) is a multifunctional food additive derived from brown seaweed, commonly used as a stabilizer, thickener, and emulsifier in processed foods. While its E-number classification (E405) often prompts skepticism from clean-label consumers, PGA is derived from alginic acid—a naturally occurring polysaccharide—and modified for enhanced solubility and function in acidic environments. For keto food manufacturers and formulators, understanding the origin, metabolic inertness, and application scope of PGA is essential for maintaining transparency and compliance with KETO Certified standards. This article examines the science, safety, and formulation role of propylene glycol alginate, evaluating its compatibility with ketogenic principles and clean label positioning.
As demand for ketogenic foods and supplements continues to grow, formulators are challenged not only by macronutrient requirements but also by the need for functional ingredients that align with clean label expectations. Among the emulsifiers and stabilizers commonly used in keto-friendly formulations, propylene glycol alginate (PGA) often sparks debate due to its synthetic-sounding name and association with the broader category of “E-number” additives.
Despite these concerns, PGA originates from alginic acid, a naturally occurring substance extracted from brown seaweed, and undergoes a controlled esterification process with propylene glycol to improve its functionality—especially in acidic, low-moisture, or high-fat systems typical of many ketogenic products.
This article provides a detailed examination of propylene glycol alginate through the lens of keto compliance, formulation utility, and consumer perception. We’ll explore how PGA performs as a low-carb-compatible thickener and stabilizer, review safety data from global regulatory agencies, and offer formulation guidance for KETO Certified products. By clarifying its classification and use cases, this resource aims to equip both product developers and health-conscious consumers with a clear, evidence-based perspective on this widely misunderstood ingredient.
What Is Propylene Glycol Alginate?
Propylene glycol alginate (PGA) is a semi-synthetic food additive derived from alginic acid, a naturally occurring polysaccharide extracted from brown seaweed species such as Laminaria and Macrocystis. To enhance its solubility and emulsifying capabilities, the alginic acid undergoes partial esterification with propylene glycol and is partially neutralized with alkaline salts, resulting in a water-dispersible compound with improved thermal and acid stability [1].
PGA is classified as an emulsifier, thickener, and stabilizer under the food additive code E405. It is widely approved by regulatory agencies including the United States Food and Drug Administration (FDA), the European Food Safety Authority (EFSA), Health Canada, and Japan’s Ministry of Health [2][3]. Due to its adaptability across pH levels and compatibility with fat-based systems, it is commonly used in:
- Oil-in-water emulsions such as salad dressings and keto condiments
- Low-pH beverages like fruit juices and electrolyte drinks
- Dairy-free and high-fat frozen desserts
- Shelf-stable baked goods and low-carb sauces
Its cold-water solubility and acid resistance make it particularly useful in ketogenic formulations where sugars, starches, and conventional thickeners are avoided.
Safety and Regulatory Status of Propylene Glycol Alginate
In the United States, PGA is classified as Generally Recognized As Safe (GRAS) under 21 CFR §184.1666, which permits its use in food products when formulated according to good manufacturing practices [2]. Internationally, the EFSA reaffirmed the safety of PGA (E405) in its 2018 re-evaluation, concluding that it poses no concern for human health at currently authorized levels of use [3].
Toxicological data from the Agency for Toxic Substances and Disease Registry (ATSDR) indicate that propylene glycol, the compound used to esterify alginic acid in PGA, is metabolized in the human liver into pyruvate and lactate—two naturally occurring intermediates of the Krebs cycle [4]. These metabolites are easily excreted and do not accumulate, even when PGA is consumed regularly.
The alginate portion of PGA, derived from seaweed, acts as a soluble dietary fiber. It is not broken down by human digestive enzymes and is excreted without contributing digestible carbohydrates or glycemic load—qualities that align PGA with ketogenic nutrition guidelines [5].
Based on its regulatory approvals, physiological inertness, and favorable safety profile, PGA is permitted in KETO Certified products as long as brands ensure proper labeling and sourcing transparency. Its inclusion can improve product performance without undermining ketogenic macronutrient integrity.
Propylene Glycol Alginate in Ketogenic Formulations
Propylene glycol alginate (PGA) plays a unique role in ketogenic product development, offering both functionality and macronutrient neutrality in applications where traditional thickeners like flour, starch, or sugar alcohols are not compatible with keto macros.
Because PGA contributes no digestible carbohydrates, it is particularly useful in:
- Emulsified sauces and dressings
- High-fat, low-carb desserts
- Electrolyte or functional beverages
- Fiber-enriched baked goods
- Vegan or dairy-free keto formulations
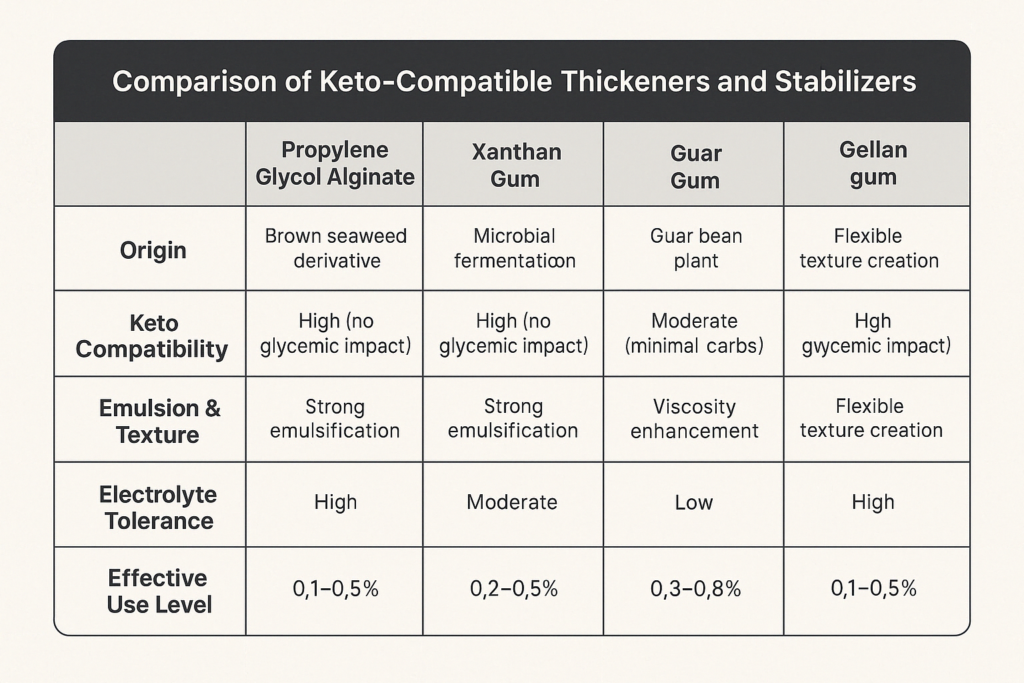
At typical usage levels of 0.1–0.5%, PGA helps stabilize emulsions, suspend particulates, and improve mouthfeel without disrupting the ketogenic macronutrient profile [6]. Its resistance to acid precipitation and tolerance for heat and ionic interactions makes it ideal for applications like keto-friendly barbecue sauce or vinaigrettes where pH and fat content are variable.
Functional Benefits in Keto Applications
- Emulsion stabilization: Supports oil-in-water stability in keto mayonnaise, aioli, and salad dressings.
- Suspension and clarity: Keeps particulates suspended in low-carb beverages and electrolyte drinks.
- Texture enhancement: Provides creaminess in frozen desserts and fat-based shakes.
- pH resilience: Maintains viscosity in acidic environments such as vinegar-based sauces or citrus blends.
Because PGA is metabolically inert and not fermented by intestinal microbiota, it avoids contributing to glycemic spikes, digestive discomfort, or insulin fluctuations—key concerns for ketogenic and diabetic populations [7].
KETO Certified Product Examples Containing PGA
KETO Certified brands utilize PGA for its ability to preserve texture, consistency, and mouthfeel such as:
- Primal Kitchen® Avocado Oil-Based Dressings – Achieve stable emulsification in clean-label vinaigrettes and creamy sauces.
For formulators, PGA offers a rare balance of clean-label compliance, keto compatibility, and functional performance. Its use in KETO Certified products is permitted under the Paleo Foundation’s standards, provided that it is clearly labeled and used within regulatory guidelines.
Clean Label Considerations and Consumer Perception
Despite its functional benefits and long-established safety profile, propylene glycol alginate (PGA) often faces skepticism from consumers seeking clean-label, minimally processed foods—particularly within the keto space, where transparency and ingredient simplicity are valued. Its E-number designation (E405) and synthetic-sounding name can lead to misconceptions, even though it is derived from natural seaweed sources and modified with widely studied food-safe compounds.
Addressing the “Chemical Name Bias”
Consumer resistance is frequently rooted in “chemical name bias”—the tendency to distrust ingredients with complex or unfamiliar names, regardless of origin or safety [8]. While PGA is partially synthesized, its alginic acid base is natural and sourced from brown seaweed. Moreover, the propylene glycol used in esterification is classified as GRAS by the FDA, and its metabolic pathway is well-understood and non-cumulative [2][4].
Educational labeling and marketing—such as clarifying that PGA is “derived from seaweed and used to stabilize texture”—can go a long way in overcoming this barrier.
Aligning with Keto Clean-Label Priorities
For keto consumers, “clean label” typically emphasizes:
- Low net carbohydrates
- No added sugars or fillers
- Transparent sourcing and function
- Non-GMO and minimal processing where possible
PGA fits these criteria when properly explained. It does not impact blood glucose, has no glycemic load, and contributes no digestible carbohydrates—features that align closely with ketogenic principles [5].
To reinforce this, brands using PGA in KETO Certified products should:
- Clearly label it by both name and function (e.g., “Propylene Glycol Alginate [Seaweed-derived stabilizer]”)
- Include it near other natural thickeners or fibers for consumer familiarity
- Use consistent terminology across digital and physical product descriptions to support search intent and SEO visibility
Competitive Advantage Through Transparent Use
Brands that embrace PGA’s functional transparency can leverage it as a competitive differentiator—especially as formulators increasingly move away from starches, maltodextrin, and sugar alcohols that often complicate keto compliance.
Used correctly, PGA allows for cleaner formulation architecture in emulsified products, shelf-stable keto snacks, and high-fat blends. When paired with MCT oil, electrolytes, and fat-soluble vitamins, it contributes to a better end-user experience—without compromising keto macros or label trust.
Formulation Synergies with Propylene Glycol Alginate in Keto Product Development
In ketogenic food and supplement innovation, achieving desirable texture, stability, and mouthfeel—without relying on sugars, starches, or high-glycemic binders—requires smart formulation. Propylene glycol alginate (PGA) offers an exceptional degree of synergy with common keto ingredients, improving product quality across categories while adhering to KETO Certified Standards.
Synergy with MCTs and Healthy Fats
Many keto formulations contain medium-chain triglycerides (MCTs), avocado oil, ghee, or other healthy fats. PGA excels in stabilizing oil-in-water emulsions, helping to:
- Prevent oil separation in dressings and sauces
- Improve creaminess in high-fat shakes
- Extend shelf life in refrigerated or RTD keto beverages
Because PGA functions well at low concentrations (as little as 0.1%), it avoids diluting the fat profile or altering caloric density—ideal for performance-based keto products.
Compatibility with Electrolytes and Mineral Salts
Products such as keto hydration powders, electrolyte tablets, and recovery beverages often contain sodium, potassium, and magnesium salts. PGA is ion-tolerant, meaning it maintains viscosity and suspension in formulations where xanthan gum or guar gum may fail under high electrolyte loads [6].
This makes PGA a valuable alternative in sports nutrition and endurance applications, particularly where clean label keto additives are required.
Low-Glycemic Sweetener Blends
In bars, baked goods, and drink mixes that use monk fruit, stevia, or erythritol, PGA supports mouthfeel and consistency. It reduces the gritty texture sometimes associated with crystalline sweeteners and improves the spreadability of reduced-sugar batters.
Additionally, because PGA does not undergo fermentation by gut bacteria, it avoids gastrointestinal issues that can arise when paired with polyols—supporting better digestive tolerance in sensitive consumers [7].
Conclusion: Propylene Glycol Alginate in the Future of Keto Innovation
As the ketogenic product landscape expands, so too does the demand for clean-label ingredients that deliver both functional and nutritional value. Propylene glycol alginate (PGA) meets this need by offering a unique combination of seaweed-derived origin, low effective dose, and metabolic neutrality—making it a standout tool in the formulation of emulsified, acidified, and texture-dependent keto products.
Despite initial concerns tied to its name or classification as an E-number additive, PGA has consistently passed toxicological review and regulatory scrutiny from leading global agencies. It does not raise blood glucose, contributes no digestible carbohydrates, and enhances product quality in categories ranging from keto salad dressings and shakes to low-carb desserts and beverages.
For brands pursuing KETO Certification, PGA presents a valuable opportunity to enhance consumer experience without compromising dietary standards. When transparently labeled and responsibly sourced, it reinforces the very principles that define successful keto innovation: clarity, efficacy, and integrity.
As formulators continue seeking alternatives to starches, gums, and fillers that can disrupt ketosis, PGA stands as a science-backed, certification-compliant solution worthy of broader adoption.
[1] U.S. Food and Drug Administration. (2024). 21 CFR §184.1666 – Propylene glycol alginate. Electronic Code of Federal Regulations.
[2] EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). (2018). Re-evaluation of propane-1,2-diol alginate (E 405) as a food additive. EFSA Journal, 16(4), 5371.
[3] Agency for Toxic Substances and Disease Registry (ATSDR). (1997). Case Study 14: Ethylene/Propylene Glycol Toxicity. In Environmental Medicine: Integrating a Missing Element into Medical Education. National Academies Press.
[4] Draget, K. I., & Taylor, C. (2011). Chemical, physical, and biological properties of alginates and their biomedical implications. Food Hydrocolloids, 25(2), 251–256.
[5] Imeson, A. (Ed.). (2010). Food Stabilisers, Thickeners and Gelling Agents. Wiley-Blackwell.
[6] Venema, K., & van den Abbeele, P. (2013). Experimental models of the gut microbiome. Best Practice & Research Clinical Gastroenterology, 27(1), 115–126.
[7] Bearth, A., Cousin, M.-E., & Siegrist, M. (2014). The consumer’s perception of artificial food additives: Influences on acceptance, risk and benefit perceptions. Food Quality and Preference, 38, 14–23.
[8] Phillips, G. O., & Williams, P. A. (Eds.). (2009). Handbook of Hydrocolloids (2nd ed.). Woodhead Publishing.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Should Xanthan Gum Be in Grain-Free, Paleo & Keto Foods?
Should Xanthan Gum Be in Grain-Free, Paleo & Keto Foods?